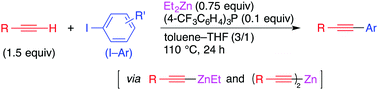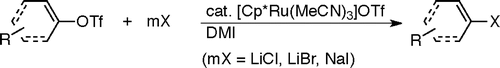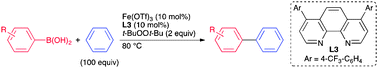投稿論文(2010年以降)
Photo-Induced α-Oxyalkylation of Aryl Chlorides with Ethers and Alcohols through Homolytic Aromatic Substitution
Aoki, K.; Yonekura,K.; Ejima, W.; Tanaka, S.; Shigeta, A.; Shirakawa, E.
Synthesis, 2024, (DOI: 10.1055/a-2273-2895).
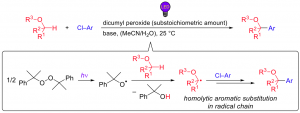
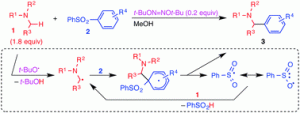
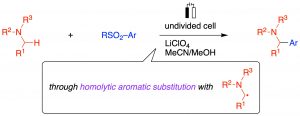
Photo-Induced α-Aminoalkylation of Sulfonylarenes with Alkylamines
Yonekura, K.; Aoki, K.; Nishida, T.; Ikeda, Y.; Oyama, R.; Hatano, S.; Abe, M.; Shirakawa, E.
Chem. Eur. J. 2023, e202302658.
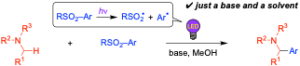
Electrochemical Direct α-Arylation of Alkylamines with Sulfonylarenes
Yonekura, K.; Murooka, M.; Aoki, K.; Shirakawa, E.
Org. Lett. 2023, 25, 6682-6687.
Manipulation of an electron by photoirradiation in the electron-catalyzed cross-coupling reaction
Shirakawa, E.; Ota, Y.; Yonekura, K.; Okura, K.; Mizusawa, S.; Sujan Kumar Sarkar; Abe, M.

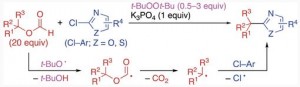
Alkylation of Heteroaryl Chlorides through Homolytic Aromatic Substitution by Alkyl Radicals Derived from Alkyl Formates
Ikeda, Y.; Mandai, T.; Yonekura, K.; Shirakawa, E.
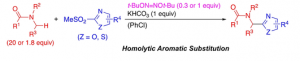
Amidoalkylation of Sulfonylheteroarenes with Alkylamides through a Radical Chain Mechanism
Ikeda, Y.; Matsukawa, Y.; Yonekura, K.; Shirakawa, E.
Eur. J. Org. Chem. 2021, 794–797.
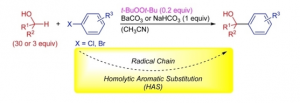
Direct α‐Arylation of Alcohols with Aryl Halides through a Radical Chain Mechanism
Aoki, K.; Yonekura, K.; Ikeda, Y.; Ueno, R.; Shirakawa, E.
Adv. Synth. Catal. 2020, 362, 2200–2204.
α-Arylation of Alkylamines with Sulfonylarenes through a Radical Chain Mechanism
Ikeda, Y.; Ueno, R.; Akai, Y.; Shirakawa, E.
Chem. Commun. 2018, 54, 10471–10474.
Electron-Catalyzed Cross-Coupling of Arylboron Compounds with Aryl Iodides
Okura, K.; Teranishi, T.; Yoshida, Y.; Shirakawa, E.
Angew. Chem., Int. Ed. 2018, 57, 7186–7190.
Highlighted in Synfacts 2018, 14, 0852.
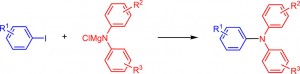
Electron-Catalyzed Coupling of Magnesium Amides with Aryl Iodides
Kiriyama, K.; Okura, K.; Tamakuni, F.; Shirakawa, E.
Chem. Eur. J. 2018, 24, 4519–4522.
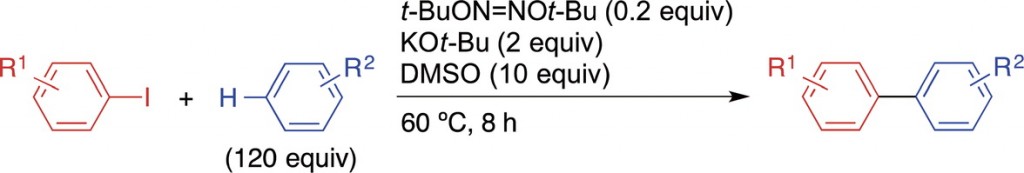
tert-Butoxide-Promoted Coupling of Aryl Iodides with Arenes Using Di-tert-butyl Hyponitrite as an Initiator
Kiriyama, K.; Shirakawa, E.
Chem. Lett. 2017, 46, 1757–1759.
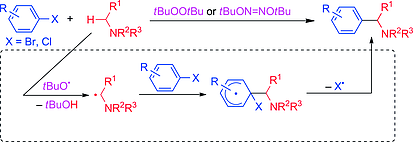
tert-Butoxy-Radical-Promoted α-Arylation of Alkylamines with Aryl Halides
Ueno, R.; Ikeda Y.; Shirakawa, E.
Eur. J. Org. Chem. 2017, 4188–4193.
Single Electron Transfer-Induced Coupling of Alkynylzinc Reagents with Aryl and Alkenyl Iodides
Okura, K.; Kawashima, H.; Tamakuni, F.; Nishida, N.; Shirakawa, E.
Chem. Commun. 2016, 52, 14019–14022.
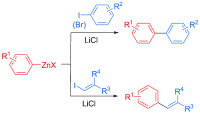
Single-Electron-Transfer-Induced Coupling of Alkylzinc Reagents with Aryl Iodides
Okura, K.; Shirakawa, E.
Eur. J. Org. Chem. 2016, 3043–3046.
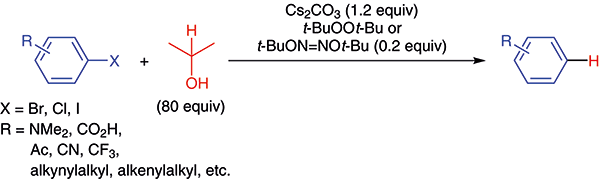
Reduction of Aryl Halides into Arenes with 2-Propanol Promoted by a Substoichiometric Amount of a tert-Butoxy Radical Source
Ueno, R.; Shimizu, T.; Shirakawa, E.
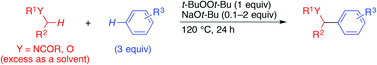
Base-Promoted Dehydrogenative Coupling of Benzene Derivatives with Amides or Ethers.
Ueno, R.; Shirakawa, E.
Org. Biomol. Chem. 2014, 12, 7469–7473.
Shirakawa, E.; Okura, K.; Uchiyama, N.; Murakami, T.; Hayashi, T.
Chem. Lett. 2014, 43, 922–924.
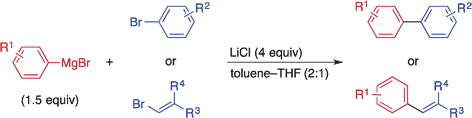
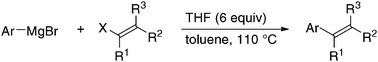
Single Electron Transfer-Induced Cross-Coupling Reaction of Alkenyl Halides with Aryl Grignard Reagents.
Shirakawa, E.; Watabe, R.; Murakami, T.; Hayashi, T.
Chem. Commun. 2013, 49, 5219–5221.
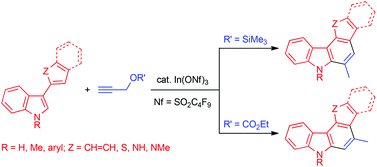
Indium-Catalyzed Annulation of 3-Aryl- and 3-Heteroarylindoles with Propargyl Ethers: Synthesis and Photoluminescent Properties of Aryl- and Heteroaryl[c]carbazoles.
Nagase, Y.; Shirai, H.; Kaneko, M.; Shirakawa, E.; Tsuchimoto, T.
Org. Biomol. Chem. 2013, 1456–1459.
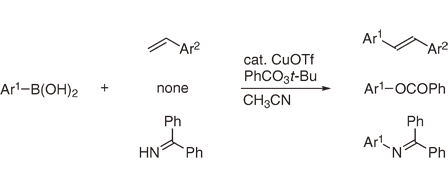
Copper-Catalyzed Oxidative C–C, C–O, and C–N Bond Forming Reactions of Arylboronic Acids.
Shirakawa, E.; Nishikawa, R.; Uchiyama, N.; Hata, I.; Hayashi, T.
Chem. Lett. 2013, 42, 269–271.
Single Electron Transfer-Induced Grignard Cross-Coupling Involving Ion Radicals as Exclusive Intermediates.
Uchiyama, N.; Shirakawa, E.; Hayashi, T.
Chem. Commun. 2013, 49, 364–366.
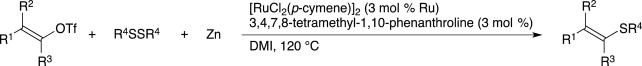
Ruthenium-Catalyzed Transformation of Aryl and Alkenyl Triflates to Halides.
Imazaki, Y.; Shirakawa, E.; Ueno, R.; Hayashi, T.
J. Am. Chem. Soc. 2012, 134, 14760–14763.
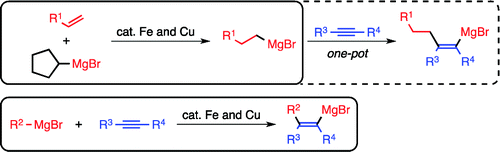
Iron–Copper Cooperative Catalysis in the Reactions of Alkyl Grignard Reagents: Exchange Reaction with Alkenes and Carbometalation of Alkynes.
Shirakawa, E.; Ikeda, D.; Masui, S.; Yoshida, M.; Hayashi, T.
J. Am. Chem. Soc. 2012, 134, 272–279.
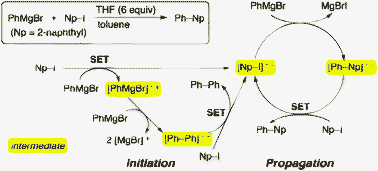
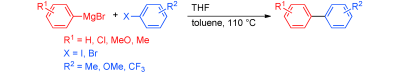
Cross-Coupling of Aryl Grignard Reagents with Aryl Iodides and Bromides through SRN1 Pathway.
Shirakawa, E.; Hayashi, Y.; Itoh, K.; Watabe, R.; Uchiyama, N.; Konagaya, W.; Masui, S.; Hayashi, T.
Angew. Chem., Int. Ed. 2012, 51, 218–221.
Ruthenium-Catalyzed Reaction of Alkenyl Triflates with Zinc Thiolates.
Imazaki, Y.; Shirakawa, E.; Hayashi, T.
Tetrahedron 2011, 67, 10212–10215.
Iron-Catalyzed Oxidative Coupling of Arylboronic Acids with Benzene Derivatives through Homolytic Aromatic Substitution Mechanism.
Uchiyama, N.; Shirakawa, E.; Nishikawa, R.; Hayashi, T.
Chem. Commun. 2011, 47, 11671–11673.
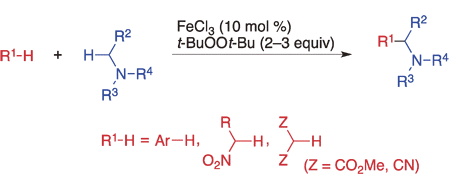
Iron-Catalyzed Oxidative Coupling of Alkylamines with Arenes, Nitroalkanes, and 1,3-Dicarbonyl Compounds.
Shirakawa, E.; Yoneda, T. Moriya, K.; Ota, K.; Uchiyama, N.; Nishikawa, R.; Hayashi, T.
Chem. Lett. 2011, 40, 1041–1043.
Iron-Catalyzed Aryl- and Alkenyllithiation of Alkynes and Its Application to Benzosilole Synthesis
Shirakawa, E.; Masui, S.; Narui, R.; Watabe, R.; Ikeda, D.; Hayashi, T.
Chem. Commun. 2011, 47, 9714–9716.
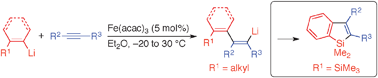
Mizoroki–Heck Type Reaction Mediated by Potassium tert-Butoxide.
Shirakawa, E.; Zhang, X.; Hayashi, T.
Angew. Chem. Int. Ed. 2011, 50, 4671–4674.
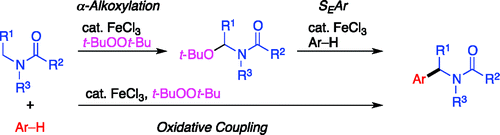
Iron-Catalyzed Oxidative Coupling of Alkylamides with Arenes through Oxidation of Alkylamides Followed by Friedel–Crafts Alkylation.
Shirakawa, E.; Uchiyama, N.; Hayashi, T.
J. Org. Chem. 2011, 76, 25–34.
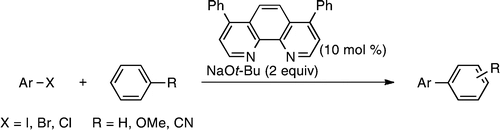
tert-Butoxide-Mediated Arylation of Benzene with Aryl Halides in the Presence of a Catalytic
1,10-Phenanthroline Derivative.
Shirakawa, E.; Itoh, K.; Higashino, T.; Hayashi, T
J. Am. Chem. Soc. 2010, 132, 15537–15539.
総説・総合論文
電子触媒クロスカップリング反応
白川英二
電子触媒クロスカップリング反応
白川英二
電子一つを触媒とするハロゲン化アリールのカップリング反応
白川英二
Transition-Metal-Free Coupling Reactions of Aryl Halides.
Shirakawa, E.; Hayashi, T.
Chem. Lett. 2012, 41, 130–134.
電子1個を触媒とするカップリング反応 —いかに遷移金属と同じように電子を操るか
白川英二
化学(化学同人),2011, 66, No. 12, 47–21.
遷移金属触媒を用いないハロゲン化アリールのカップリング反応
白川英二
Organometallic News, 2016, No. 3, 98–103.
著書
「ウォーレン有機化学」第2版(上)・(下)
Clayton, J.; Greeves, N.; Warren, S.; Wothers, P. 著, 野依良治, 奥山 格, 柴崎正勝, 檜山爲次郎 監訳, 白川英二 他 訳
東京化学同人: 東京, 2015.
「有機合成化学」
檜山爲次郎, 大嶌幸一郎 編著, 丸岡啓二, 高井和彦, 松原誠二郎, 野崎京子, 白川英二, 忍久保洋 著
東京化学同人: 東京, 2012.
※リンク先で「研究活動」を選択してください。








![[CREST] アニオンラジカル制御が拓く革新的電子触媒系](http://www.kg-applchem.jp/shirakawa/wp-content/uploads/sites/16/2019/06/crest-ja120.png)