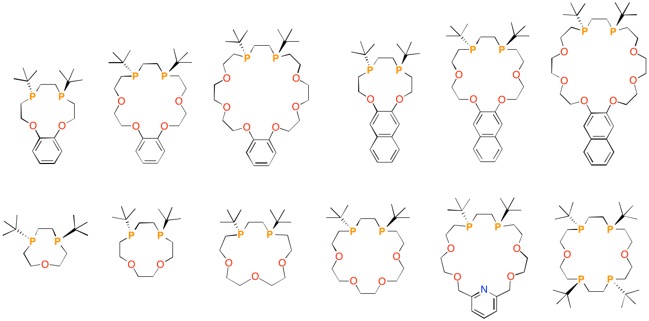
Unlike nitrogen atoms, the trivalent phosphorus atom in phosphine (PR3) can act as a chiral center due to its high inversion energy. In addition, phosphines have a lone pair and vacant d orbitals; therefore, phosphines show good coordination ability toward transition metals. Hence, phosphines are widely used as ligands for transition metals. From this viewpoint, various P-stereogenic phosphines have been synthesized and are very useful as chiral ligands for transition metal-catalyzed asymmetric reactions. Recently, we have focused on P-stereogenic phosphines and synthesized optically active polymers [1], oligomers [2], and cyclic compounds [3] containing these units in the main skeleton. In particular, a practical synthetic route to P-stereogenic diphosphacrowns has been developed [4].
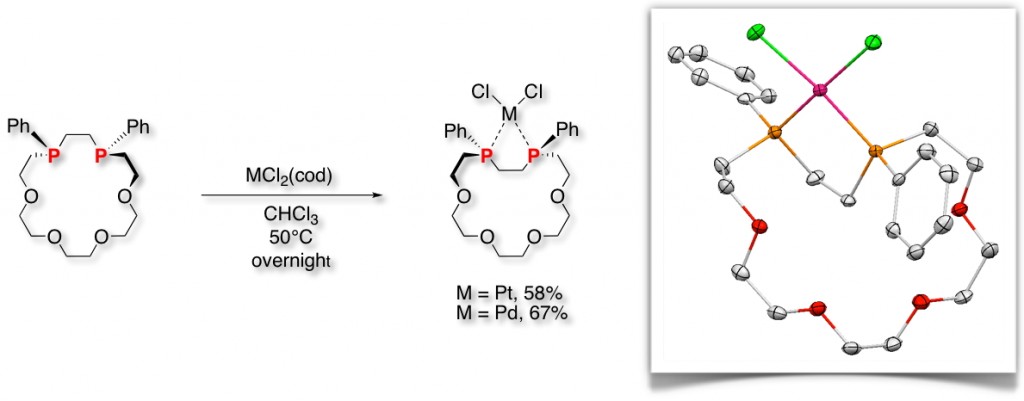
Since the first report on the synthesis of dibenzo-18-crown-6 by Pedersen, numerous other crown ether derivatives have been reported. However, to our knowledge, there have been no examples of the synthesis of P-stereogenic phosphacrowns possessing chiral phosphorus atoms in the ring skeletons. The prepared P-stereogenic diphosphacrowns have a chiral ring structure as well as chiral heteroatoms (phosphorus atoms) that interact directly with guest ions and molecules, leading to the new type of optically active molecule [4].
References
- For example, see: Imoto, H.; Morisaki, Y.; Chujo, Y. Chem. Commun. 2010, 46, 7542-7544.
- For example, see: Morisaki, Y.; Ouchi, Y.; Naka, K.; Chujo, Y. Chem. Asian J. 2007, 2, 1166-1173.
- (a) Morisaki, Y.; Ouchi, Y.; Fukui, T.; Naka, K.; Chujo, Y. Tetrahedron Lett. 2005, 46, 7011-7014. (b) Morisaki, Y.; Imoto, H.; Ouchi, Y.; Nagata, Y.; Chujo, Y. Org. Lett. 2008, 10, 1489-1492. (c) Morisaki, Y.; Imoto, H.; Kato, R.; Ouchi, Y.; Chujo Y. Heterocycles 2012, 85, 2543-2550.
- (a) Morisaki, Y.; Imoto, H.; Tsurui, K.; Chujo, Y. Org. Lett. 2009, 11, 2241-2244. (b) Morisaki, Y.; Imoto, H.; Hirano, K.; Hayashi, T.; Chujo Y. J. Org. Chem. 2011, 76, 1795-1803. (c) Morisaki, Y.; Kato, R.; Chujo Y. J. Org. Chem. 2013, 78, 2769-2774. (d) Kato, R.; Morisaki, Y.; Chujo Y. Asian J. Org. Chem. 2015, 4, 1410-1416. (e) Kato, R.; Watanabe, H.; Morisaki, Y.; Chujo Y. Heterocycles 2015, 91, 2295-2306. (f) Morisaki, Y.; Kato, R.; Chujo Y. ChemistryOpen 2016, 5, 325-330.