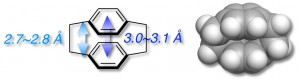
[2.2]Paracyclophane is a unique aromatic compound consisting of two stacked benzene rings in close proximity (approximately 3.0 Å). When the [2.2]paracyclophane skeleton is incorporated into a π-conjugated backbone, π-stacked structure, in which π-conjugated systems are partly stacked and aligned, can be formed [1].
The representative examples of [2.2]paracyclophane-containing π-stacked systems are as follows…
1.Synthesis of through-space conjugated oligomers and polymers
Incorporation of a [2.2]paracyclophane skeleton into a π-conjugated polymer and oligomer backbones leads to a π-stacked structure [1,2]. We have prepared oligomers, in which 2~10 π-electron systems are partly π-stacked in proximity in their main chains, and investigated the optical properties [2a]. Their band-gap energies decreased gradually as the number of the repeating π-stacked systems increased, indicating the extension of the π-conjugation length via the through-space interaction. Such a conjugation system via the through-space interaction is the through-space conjugation system.
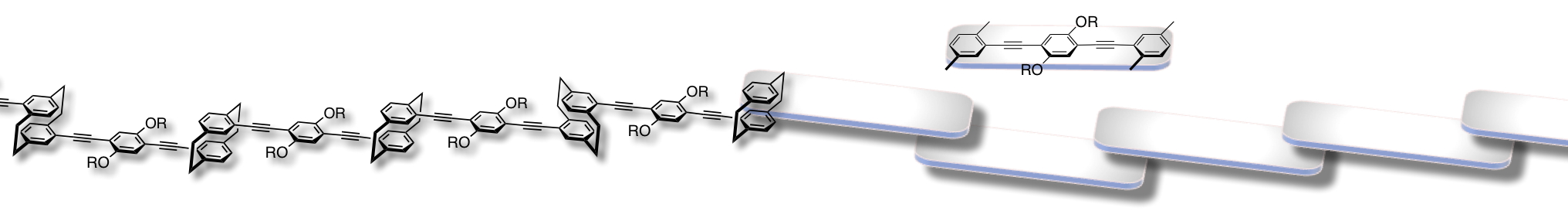
We disclosed the unique properties of the through-space conjugated systems in the ground and excited states. Hole-transporting ability of the through-space conjugated system was higher than that of common through-bond conjugated systems.
[1] For example, see: (a) Morisaki, Y.; Chujo, Y. Angew. Chem. Int. Ed. 2006, 45, 6430-6437.
(b) Morisaki, Y.; Chujo Y. Polym. Chem. 2011, 2, 249-1257.
(c) Morisaki, Y.; Chujo Y. Chem. Lett. 2012, 41, 840-846.
(d) In π-Stacked Polymers and Molecules: Synthesis, Properties, and Theory, Springer, Berlin; 2014, Chapter 3, pp 151-184.
[2] (a) Morisaki, Y.; Ueno, S.; Saeki, A.; Asano, A.; Seki, S.; Chujo Y. Chem. Eur. J. 2012, 18, 4216-4224.
(b) Morisaki, Y.; Ueno, S.; Chujo Y. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 334-339.
2.Molecular wires that transfer informations unidirectionally
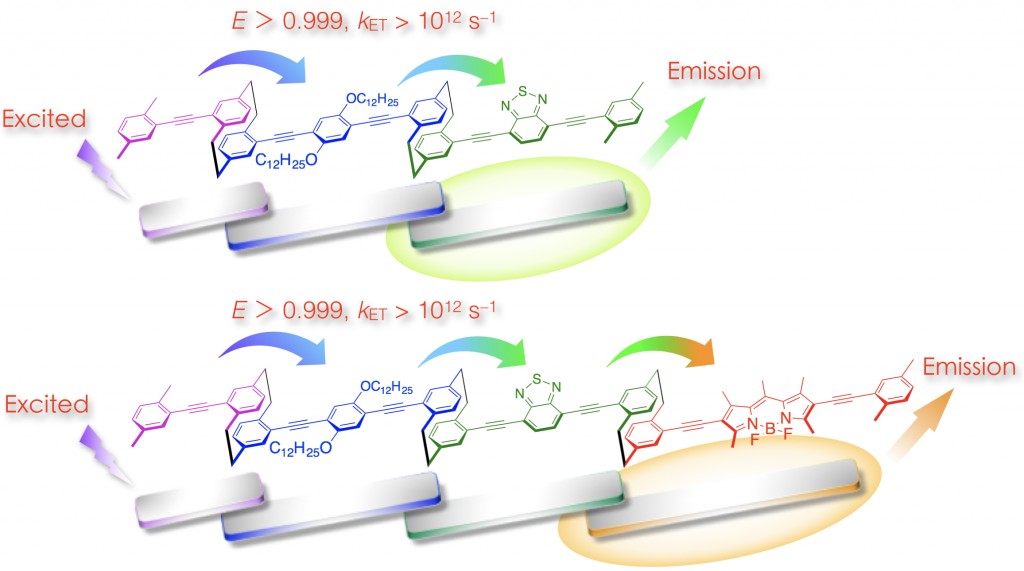
Each partially stacked π-electron system in the through-space conjugated systems absorbs and emits light independently. We have synthesized [2.2]paracyclophane-containing through-space conjugated dimers and trimers [3]. It was found that photoexcited energy as transferred through the stacked π-electron systems in a single polymer chain. The [2.2]paracyclophane scaffold appropriately aligned the transition dipole moments of the stacked π-electron systems, resulting in a high energy-transfer efficiency (FET > 0.999) with a high energy-transfer rate constant (kET) on the order of 1012 s–1. Using this system, an efficient unidirectional energy transfer could be achieved [3].
[3] (a) Morisaki, Y.; Kawakami, N.; Nakano, T.; Chujo Y. Chem. Eur. J. 2013, 19, 17715-17718.
(b) Morisaki, Y.; Kawakami, N.; Nakano, T.; Chujo Y. Chem. Lett. 2014, 43, 426-428.
(c) Morisaki, Y.; Kawakami, N.; Shibata, S.; Chujo Y. Chem. Asian J. 2014, 9, 2891-2895.
(d) Morisaki, Y.; Shibata, S.; Chujo Y. Can. J. Chem. 2017, 95, 424-431 (DOI: 10.1139/cjc-2016-0526).
3.Synthesis of Polycyclic Aromatic Hydrocarbon (PAH)-Stacked Molecules
We have synthesized π-stacked molecules in which phenanthrene [4a,b], benzo[c]phenanthrene [4a,b], and triphenylene [4c] as polycyclic aromatic hydrocarbons (PAHs) are stacked, from disubstituted or tetrasubstituted [2.2]paracyclophanes. PAH unis were twisted by steric hindrance between the aromatic ring and the bridge methylene in [2.2]paracyclophane. One-handed helical structures were induced in phenanthrene- and benzo[c]phenanthrene-stacked molecules by planar chirality of [2.2]paracyclophane, leading to emission of circularly polarized luminescence (CPL).
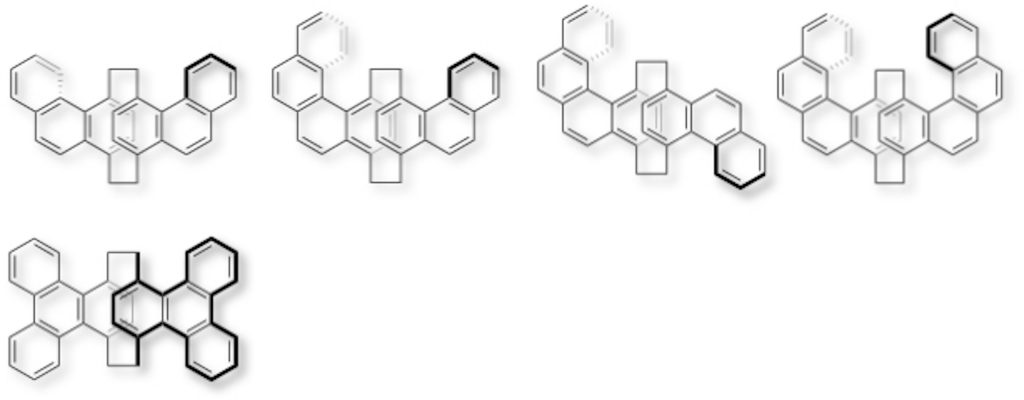
[4] (a) Tsuchiya, M.; Maeda, H.; Inoue; R.; Morisaki, Y. Chem. Commun.2021, 57, 9256-9259 .
(b) Yanagawa, A.; Tsuchiya, M.; Inoue; R.; Morisaki, Y. J. Mater. Chem. C 2023, 11, 986-993.
(c) Yanagawa, A.; Inoue; R.; Morisaki, Y.Chem. Lett. 2024, 53, upad018.
4.Conjugated microporous polymers
We have prepared conjugated microporous polymers (CMPs) containing tetrasubstituted [2.2]paracyclophane units as the junctions of the network structure [5]. The CMPs comprised micropores with diameters of less than 2 nm. They exhibited large surface areas; the BET surface area (SBET value) of the CMP synthesized by Yamamoto coupling reached 1000 m2/g. The morphology of this CMP was relatively uniform and the particles had diameters of approximately 200 nm; therefore, it was readily dispersed in common organic solvents. The polymer networks can be tuned by introducing various aromatic units into the cyclophane monomer. Studies on application of the CMPs consisting of tetrasubstituted [2.2]paracyclophane junctions as host materials for light-harvesting antennae are underway.
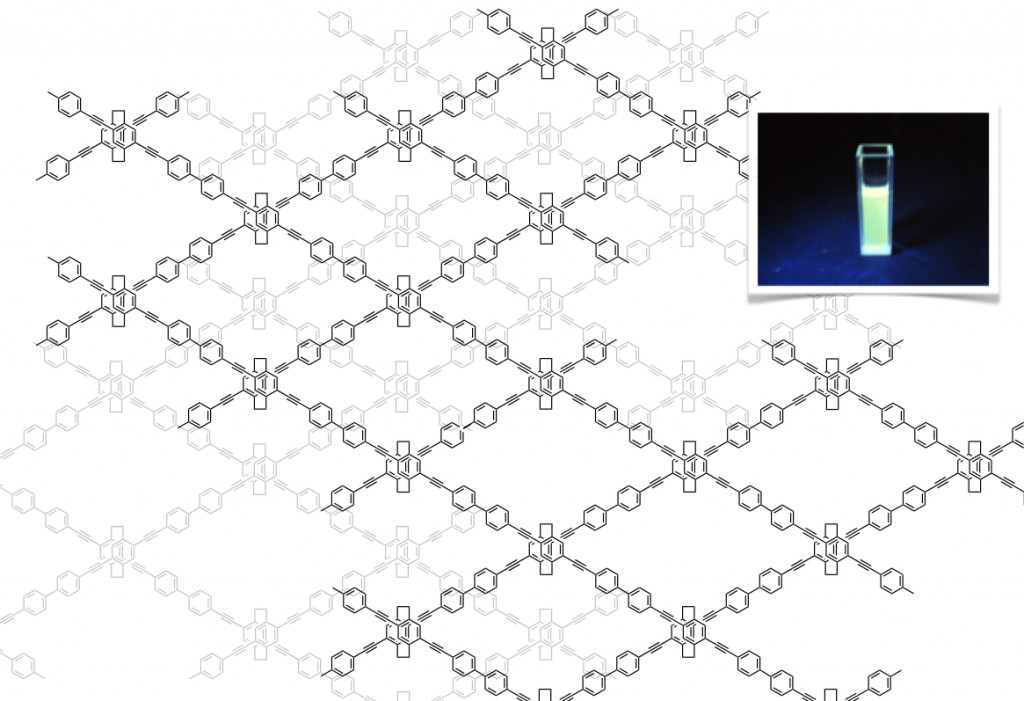
[5] (a) Morisaki, Y.; Gon, M.; Tsuji, Y.; Kajiwara, Y.; Chujo Y. Tetrahedron Lett. 2011, 52, 5504-5507.
(b) Morisaki, Y.; Gon, M.; Tsuji, Y.; Kajiwara, Y.; Chujo Y. Macromol. Chem. Phys. 2012, 213, 572-579.
(c) Morisaki, Y.; Gon, M.; Chujo Y. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 2311-2316.