Excellent CPL-emitters : Planar Chiral Cyclophane Chemistry
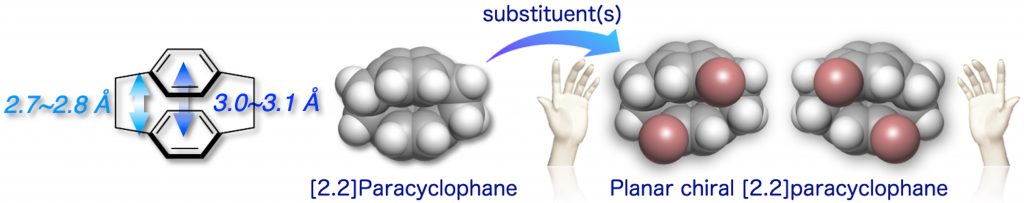
[2.2]Paracyclophane is a unique aromatic compound consisting of two stacked benzene rings in proximity. Planar chirality, which arises from the proximally fixed benzene rings, is one of the interesting structural characteristics of the [2.2]paracyclophane skeleton. We reported practical methods for the optical resolution of planar chiral 4,12-disubstituted and 4,7,12,15-tetrasubstituted [2.2]paracyclophane isomers [1] below.
We have synthesized planar chiral [2.2]paracyclophane-based conjugated molecules. We reported the first observation of chiral luminescence “circularly polarized luminescence = CPL” from the planar chiral molecules in the world [2,3].
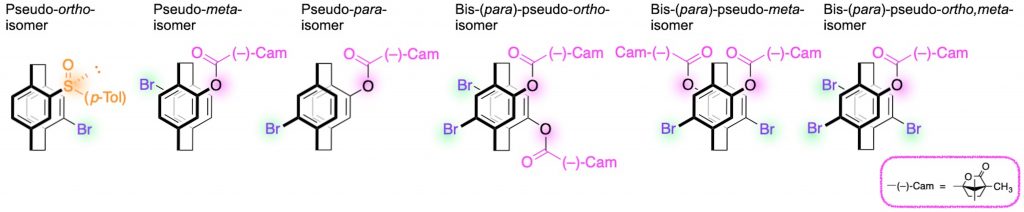
CPL active molecules are the promising candidates for 3D light-emitting-devices, liquid crystals, dyes for forgery-prevention of documents, and so on. The representative examples of CPL emitters based on the planar chiral [2.2]paracyclophane skeletons are as follows…
[1] (a) Pseudo-ortho-disubstituted [2.2]paracyclophane: Chem. Lett. 2012, 41(9), 990-992.
(b) Pseudo-meta-disubstituted [2.2]paracyclophane: Chem. Commun. 2021, 57(73), 9256-9259.
(c) Pseudo-para-disubstituted [2.2]paracyclophane: J. Mater. Chem. C 2023, 11(3), 986-993.
(d) Bis-(para)-pseudo-ortho-tetrasubstituted [2.2]paracyclophane: Chem. Asian J. 2019, 14(10), 1681-1685.
(e) Bis-(para)-pseudo-meta-tetrasubstituted [2.2]paracyclophane: Chirality 2018, 30(10), 1109-1114.
(f) Bis-(para)-pseudo-ortho,meta-tetrasubstituted [2.2]paracyclophane: J. Am. Chem. Soc. 2014, 136(9), 3350-3353.
[2] Polym. Chem. 2012, 3(10), 2727-2730. [3] Recent Accounts: (a) Bull. Chem. Soc. Jpn. 2019, 92(2), 265-274.
(b) In Circularly Polarized Luminescence of Isolated Small Organic Molecules, Springer, Singapore; 2020, Chapter 3, pp 31-52.
(c) In Progress in the Science of Functional Dyes, Springer, Singapore; 2021, Chapter 10, pp 343-374.
1.CPL-emitters consisting of planar chiral disubstituted [2.2]paracyclophanes
Optically active V-, N-, M-, triangle-shaped oligomers [4] and zig-zag-shaped polymers [2,5] were prepared using planar chiral disubstituted [2.2]paracyclophane building blocks.
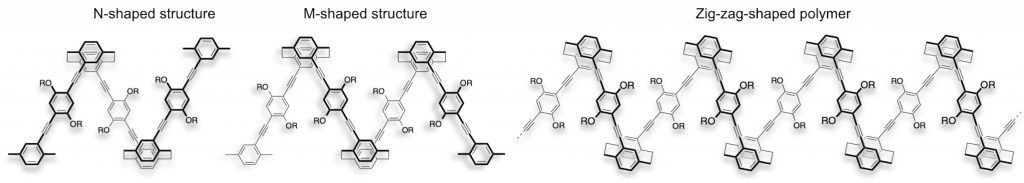
All molecules emit bright CPL. We found that the oligomers and polymers folded to form one-handed helical structures in the excited state.
[4] Chem. Eur. J. 2014, 20(27), 8386-8390. [5] (a) Polym. J. 2015, 47(3), 278-281.
(b) Polym. J. 2023, 55, 537-545.
2.CPL-emitters consisting of planar chiral tetrasubstituted [2.2]paracyclophanes
We succeeded in the optical resolutions of planar chiral tetrasubstituted [2.2]paracyclophanes and in the syntheses of chiral molecules with unique optically active second-ordered structures, such as X-shaped molecules [6], dendrimers [7], one-handed double helix [8], cyclic oligomers [9], #-shaped cyclic tetramer [10],†-shaped molecules, and ‡-shaped molecules [11]. They also emit bright CPL.
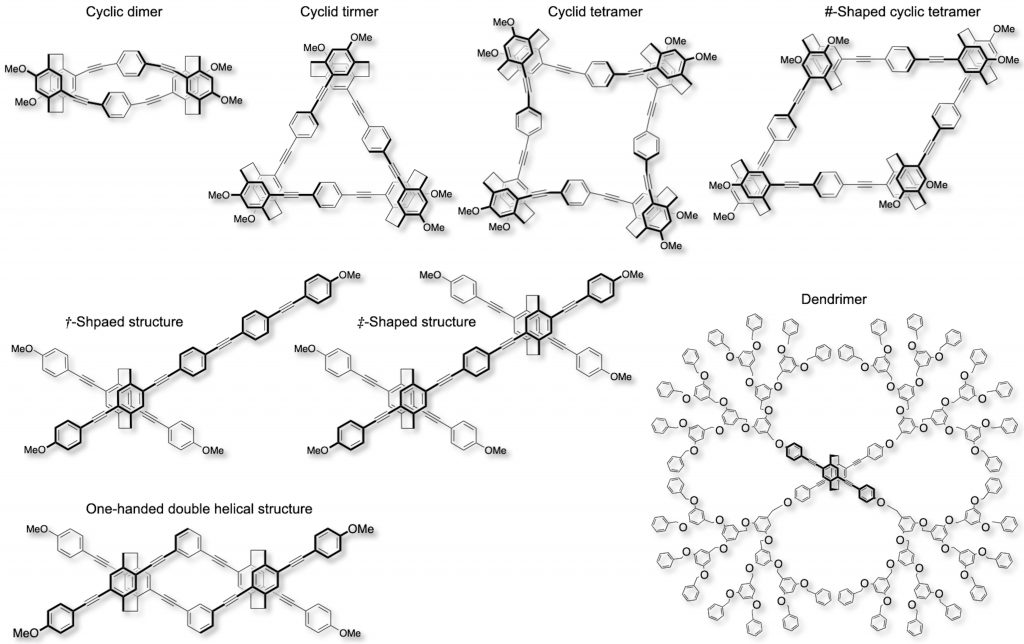
[6] (a) Eur. J. Org. Chem. 2015, (35), 7756-7762.
(b) Chem. Eur. J. 2017, 23(26), 6323-6329.
(c) Macromolecules 2017, 50(5), 1790-1802.
(d) Macromolecules 2017, 50(5), 1790-1802. [7] Chem. Eur. J. 2016, 22(7), 2291-2298. [8] Chem. Asian J. 2016, 11(18), 2524-2527. [9] Chem. Asian J. 2022, 17(2), e202101267(1-7). [10] Chem. Eur. J. 2023, 29(18), e202203533. [11] Bull. Chem. Soc. Jpn. 2020, 93(10), 1193-1199.
3.Cyclic molecules emitting CPL with high anisotropy factors
We synthesized optically active propeller-shaped cyclic molecules [1f,12] and one-handed helical molecules [13]. They emit intense CPL with high anisotropy factors (high |glum| values) of the order of 10^–2 and BCPL value over 100 M–1cm–1. We disclosed experimentally and theoretically that optically active cyclic and helical molecules with a single π-conjugated system emit excellent CPL with high |glum| values.
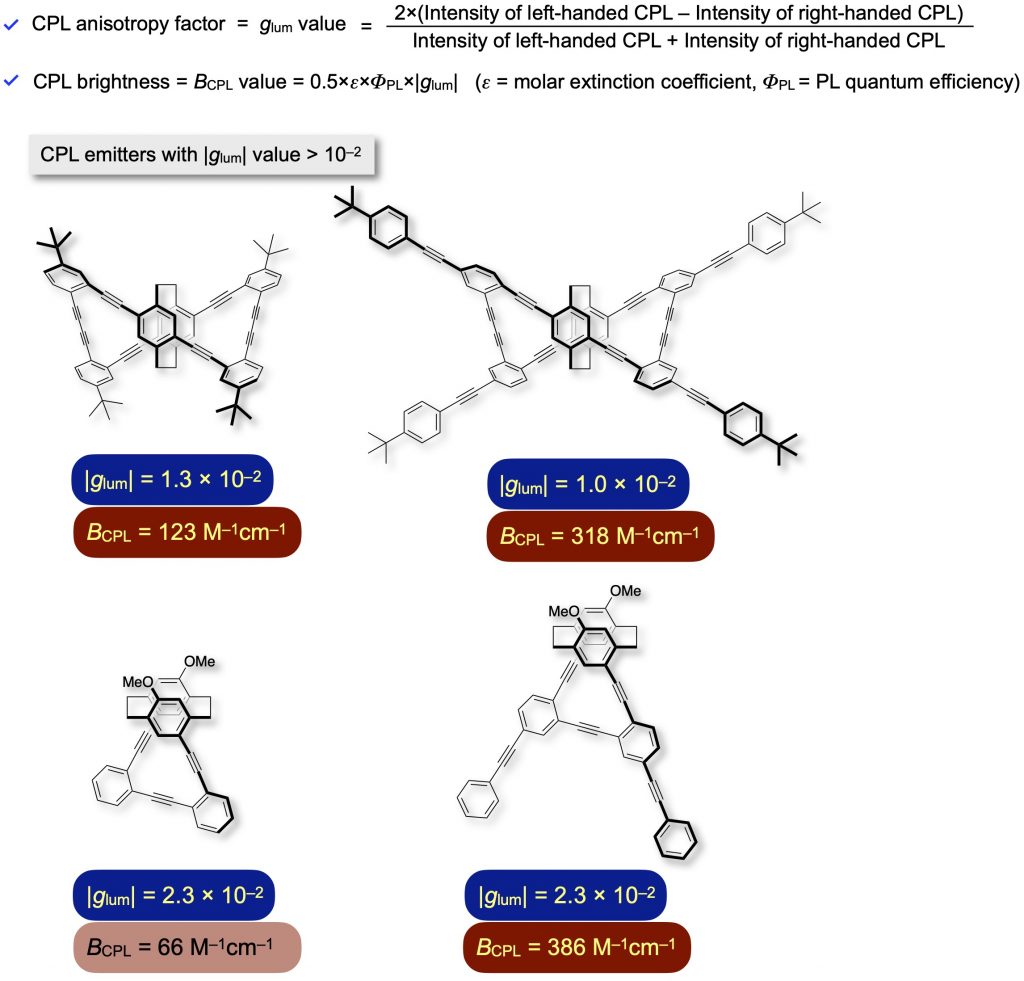
[12] (a) J. Mater. Chem. C 2015, 3(3), 521-529.
(b) Asian J. Org. Chem. 2016, 5(3), 353-359. [13] (a) Bull. Chem. Soc. Jpn. 2022, 95(1), 110-115.
(b) Adv. Funct. Mater. 2024, in press. DOI: 10.1002/adfm.202310566
4.Control of axial chirality, helicity, and twist chirality by planar chirality
Axial chirality [14], helicity [1b,1c,13,14b,15], and twist chirality [15] were controlled by planar chirality of the [2.2]paracyclophane skeletons. Intense CPL was observed from all molecules. Dual emission was observed from molecules with the controlled axial chirality [14a]. Axial chiralities of o-quinquephenyl-stacked double-layered molecule were controlled to form one-handed helical structure, which exhibited intense CPL [14b]. Helicity was induced to phenanthrene to be [3]helicene by the planar chiral [2.2]paracyclophane [1b]. Greater degree of anthracene twisting resulted in a higher CPL |glum| value [15].
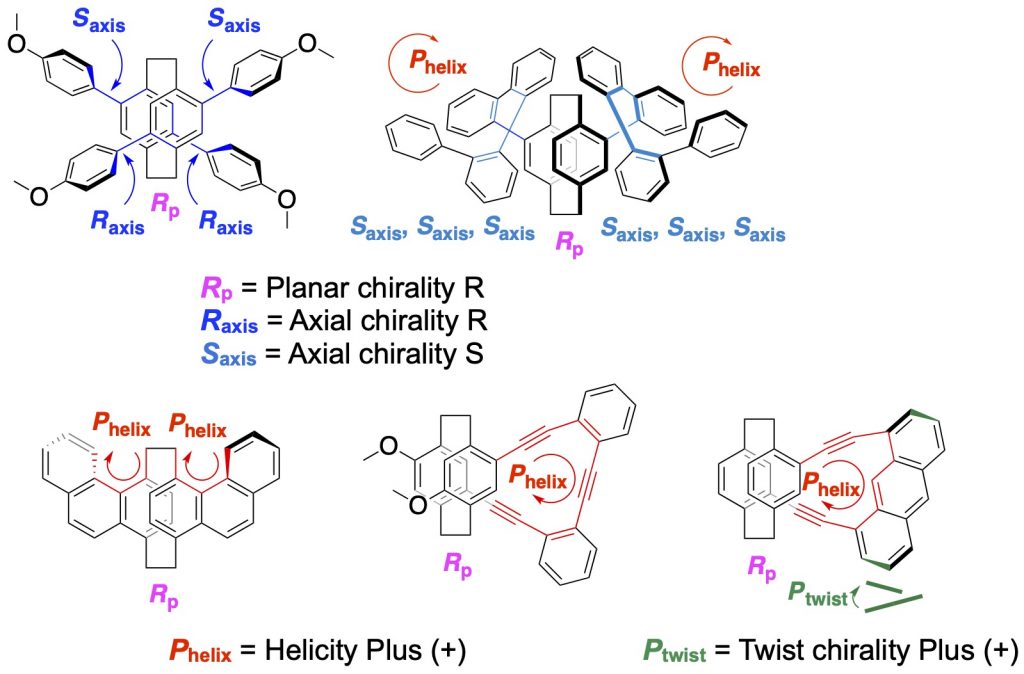
[14] (a)Chem. Eur. J. 2020, 26(65), 14871-14877.
(b) Chem. Commun. 2024, 60(11), 1468-1471. [15] Chem. Asian J. 2022, 17(15), e202200418(1-6).
5.Systematic syntheses of optically active π-staked molecules: correlation between orientation of the stacked π-electron systems and CPL
π-Stacked molecules become planar chiral molecules depending on the orientation of the two stacked π-electron systems. We have investigated correlation between orientation of the stacked π-electron systems and CPL by changing stacked angles (60° or 120°), stacking positions (center or terminal), position of donor units, and number of donor units in the two π-electron systems [16].
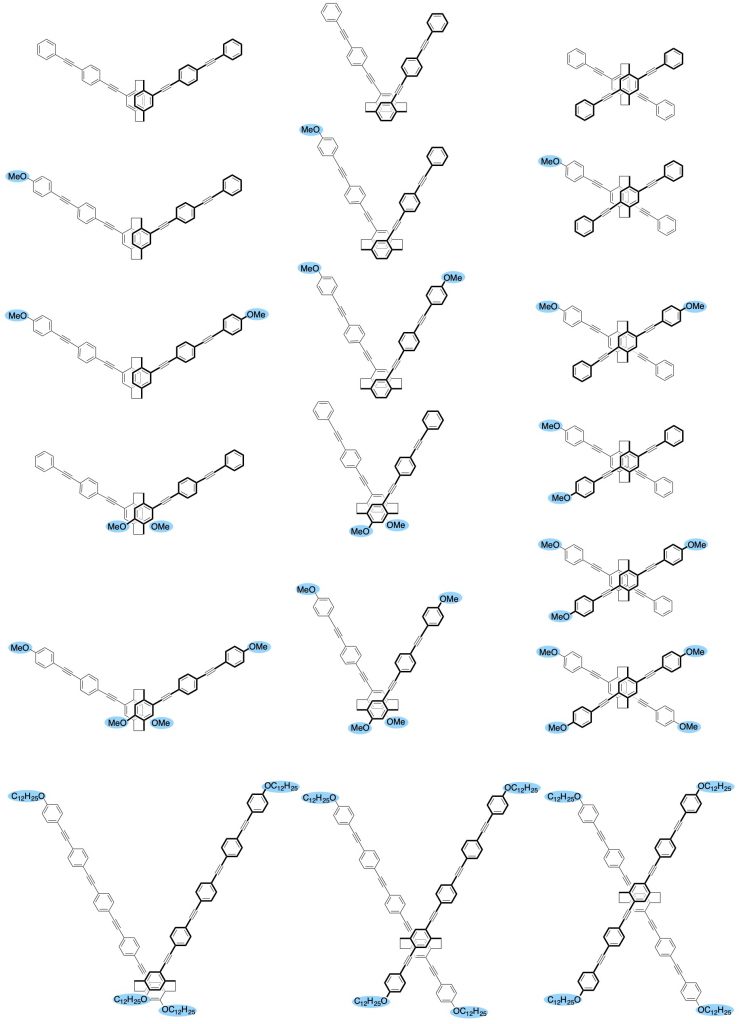
[16] (a) Mater. Chem. Front. 2018, 2(4), 791-795.
(b) Bull. Chem. Soc. Jpn. 2021, 94(2), 451-453.
(c) ChemistrySelect 2021, 6(45), 12970-12974.
(d) Eur. J. Org. Chem. 2021, 5725-5731.
(e) Bull. Chem. Soc. Jpn. 2022, 95(4), 595-601.
(f) Bull. Chem. Soc. Jpn. 2022, 95(9), 1353-1359.
(g) Tetrahedron 2023, 138, 133406(1-6).
(h) ChemistrySelect 2023, 8(26), e202301844(1-5).